These guidelines must be evaluated in context of the WHO Guidelines which state: "Infectious diseases caused by pathogenic bacteria, viruses, protozoa, and helminths are the most common and widespread health risk associated with drinking-water" (Chapter 7, Microbiological Aspects; Section 7.1, pg 118). Additionally, a previous version of these guidelines states: "Where local circumstances require that a choice must be made between meeting either microbiological guidelines or guidelines for disinfectants or disinfectant by-products, the microbiological quality must always take precedence, and where necessary, a chemical guideline value can be adopted corresponding to a higher level of risk. Efficient disinfection must never be compromised" (Chemical Aspects; Section 3.6.4, pg 49/65).
In disinfection, gaseous chlorine (Cl2) or liquid sodium hypochlorite (bleach, NaOCl) is added to, and reacts with, water to form hypochlorous acid. In the presence of bromine, hypobromous acid is also formed. Both chlorine and bromine are in the “halogen” group of elements, and have similar chemical characteristics. Hypochlorous and hypobromous acid form strong oxidizing agents in water and react with a wide variety of compounds, which is why they are such effective disinfectants.
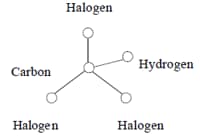
In 1974, Rook discovered that hypochlorous acid and hypobromous acid also react with naturally occurring organic matter to create many water disinfection by-products, including the four primary trihalomethanes:
- Chloroform – CHCl3
- Bromodichloromethane (BDCM) – CHCl2Br
- Dibromochloromethane (DBCM) – CHClBr2
- Bromoform – CHBr3
At the center of each of the four trihalomethanes is a carbon atom, and it is surrounded by and bound to four atoms: one hydrogen and three halogens. These four compounds are collectively termed trihalomethanes and are abbreviated as either THM or TTHM (for total trihalomethanes).
Rook’s discovery of THMs in drinking water led to research on other chemicals formed when chlorine is added to water, and to the health effects of these chemicals. Richardson identified greater than 600 water disinfection by-products in chlorinated tap water, including haloacetic acids (HAAs). THMs, and to a lesser extent HAAs, are currently used as indicator chemicals for all potentially harmful compounds formed by the addition of chlorine to water. In many countries the levels of THMs and HAAs in chlorinated water supplies are regulated based on this assumption.
Humans are exposed to DBPs through drinking-water and oral, dermal, and inhalational contact with chlorinated water 6. In populations who take hot showers or baths, inhalation and dermal absorption in the shower accounts for more exposure to THMs than drinking water .
World Health Organization (WHO) Research and Guideline Values for DBPs
The World Health Organization (WHO) International Agency for Research on Cancer (IARC) reviews research conducted on potential carcinogens and develops monographs that summarize the research and classify the compound. Links to the monographs for BDCM, DBCM, bromoform, and chloroform are available below (see Additional Resources(https://www.cdc.gov/safewater/chlorination-byproducts.html#resources) ). As can be seen in Table 1 (below), chloroform and BDCM are classified as possible human carcinogens. The classifications of possible human carcinogens come from data that is extrapolated from research on animals that may or may not be relevant to human cancer. DBCM and bromoform are not classifiable, indicating there is no evidence supporting these two compounds as carcinogens, but there is not enough research to classify them as non-carcinogenic. There is inadequate epidemiological evidence of carcinogenicity in humans for all four compounds.
| Table 1: IARC Classification of THMs | ||
|---|---|---|
| Humans | Classification | |
| Chloroform | Inadequate evidence for human carcinogenicity. | Possible human carcinogen (Group 2B) |
| Bromodichloromethane | Inadequate evidence for human carcinogenicity. | Possible human carcinogen (Group 2B) |
| Dibromochloromethane | Inadequate evidence for human carcinogenicity. | Not classifiable as to its carcinogenicity in humans (Group 3) |
| Bromoform | Inadequate evidence for human carcinogenicity. | Not classifiable as to its carcinogenicity in humans (Group 3) |
WHO states that “all people, whatever their stage of development and their social and economic conditions, have the right to have access to an adequate supply of safe drinking water” . To this end, WHO has developed guideline values for many contaminants in drinking water. It is important to note that these guideline values are not standards. “It must be emphasized that the guideline values recommended are not mandatory limits. In order to define such limits, it is necessary to consider the guideline values in the context of local or national environmental, social, economic, and cultural conditions and waterborne disease occurrence” .
To develop the guideline values for drinking-water, WHO reviewed the literature for well-designed and documented studies showing health effects from exposure to each of the THMs . A safety factor of 1,000, an average adult human weight of 60 kilograms, and an average drinking water consumption of 2 liters per day were incorporated into the development of each guideline value. The chloroform, bromoform, and dibromochloromethane guideline values were all obtained using a total daily intake calculation. It was assumed that 50 percent of total daily intake of chloroform came from drinking water, and 20 percent of total daily intake of bromoform and dibromochloromethane came from drinking water (in areas with no showers, this assumption leads to a conservative estimate of risk). The models developed for bromodichloromethane and chloroform were based on an excess cancer risk of 10-5, or one extra cancer per 100,000 people at the guideline value for 70 years .
- The chloroform guideline value was developed from a study showing hepatotoxicity in beagle dogs ingesting chloroform-laced toothpaste for 7.5 years. (A linearized multi-stage model based on observed increases in kidney tumors in male rats supports this total daily intake calculation).
- The bromoform guideline value was developed from a study showing lesions on the livers of rats exposed to bromoform for 90 days.
- The dibromochloromethane guideline value was developed based on the absence of histopathological effects in rats exposed for 90 days.
- The bromodichloromethane guideline value was developed using a linearized multi-stage model based on observed increases in kidney tumors in male mice.
The WHO Guideline Values for the THMs are shown in Table 2. WHO also considers potential health effects caused by exposure to the four compounds simultaneously. In addition to the individual guidelines, there is an additional guideline that states the following: the sum of each individual THM concentration divided by its guideline value cannot be greater than one. This is depicted in the following equation:
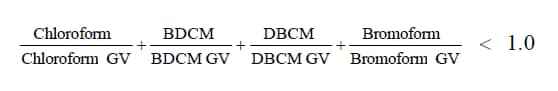
| Table 2: WHO Guideline Values for Trihalomethanes in Drinking Water (WHO, 1996) | |
|---|---|
| WHO Guideline Value | |
| Chloroform | 200 μg/L |
| Bromodichloromethane | 60 μg/L |
| Dibromochloromethane | 100 μg/L |
| Bromoform | 100 μg/L |
These guidelines must be evaluated in context of the WHO Guidelines which state: "Infectious diseases caused by pathogenic bacteria, viruses, protozoa, and helminths are the most common and widespread health risk associated with drinking-water" (Chapter 7, Microbiological Aspects; Section 7.1, pg 118).
Most importantly, the WHO specifically states in the 2nd edition of the Guidelines that: "Where local circumstances require that a choice must be made between meeting either microbiological guidelines or guidelines for disinfectants or disinfectant by-products, the microbiological quality must always take precedence, and where necessary, a chemical guideline value can be adopted corresponding to a higher level of risk. Efficient disinfection must never be compromised" (Chemical Aspects; Section 3.6.4, pg 49/65). In the 4th edition of the Guidelines, the WHO states: "In all circumstances, disinfection efficiency should not be compromised in trying to meet guidelines for DBPs, including chlorination by-products, or in trying to reduce concentrations of these substances" (Chapter 8 Chemical Aspects, Section 8.5.4, pg 188).
Thus, waterborne pathogens pose a real and more immediate threat to health; water disinfection by-products are certainly the lesser of these two evils.
Most importantly, the WHO specifically states in the 2nd edition of the Guidelines that: "Where local circumstances require that a choice must be made between meeting either microbiological guidelines or guidelines for disinfectants or disinfectant by-products, the microbiological quality must always take precedence, and where necessary, a chemical guideline value can be adopted corresponding to a higher level of risk. Efficient disinfection must never be compromised" (Chemical Aspects; Section 3.6.4, pg 49/65). In the 4th edition of the Guidelines, the WHO states: "In all circumstances, disinfection efficiency should not be compromised in trying to meet guidelines for DBPs, including chlorination by-products, or in trying to reduce concentrations of these substances" (Chapter 8 Chemical Aspects, Section 8.5.4, pg 188).
Thus, waterborne pathogens pose a real and more immediate threat to health; water disinfection by-products are certainly the lesser of these two evils.
USEPA Standards for DBPs
The disinfectant/disinfection by-products (D/DBP) rule that regulates DBPs in the United States was designed to be implemented in three stages (Table 3) , . The US Environmental Protection Agency (USEPA) does not regulate THMs or HAAs individually – there is only a standard for total THMs and total HAAs.
| Table 3: D/DBP Rule Implementation, USEPA | ||
|---|---|---|
| Stage | TTHM Standard | HAA Standard |
| Initial | 100 μg/L | |
| Stage 1 | 80 μg/L | 60 μg/L |
| Stage 2 | 80 μg/L | 60 μg/L |
The USEPA has calculated cancer potency factors for the four THMs, which can be used to calculate the probability of cancer for varying exposure levels (Table 4). As can be seen, DBCM has the highest factor, and bromoform is an order of magnitude lower.
| Table 4: USEPA Cancer Potency Factors | |
|---|---|
| Compound | Cancer Potency Factor |
| Chloroform | insufficient data |
| Bromodichloromethane | 0.062 mg/kg/day |
| Dibromochloromethane | 0.084 mg/kg/day |
| Bromoform | 0.0079 mg/kg/day |
Thus, the extra cancer from chloroform was calculated to be negligible.
Other countries in the developed world, particularly in Europe, have established much stricter standards for DBPs in drinking water. These countries have the resources to follow the precautionary principle, which advocates the avoidance of chemicals until they are proven safe. These low standards are met, in part, by researching and implementing alternative disinfection methods (such as the use of ozone, UV light, and chloramines) and water treatment strategies (such as filtration before disinfection).
DBPs and the Safe Water System
Addition of chlorine to untreated water will lead to the formation of DBPs. A significant amount of energy and time has been invested in the United States and Europe to determine the human health effects of these DBPs and how to restructure water treatment processes to prevent DBP formation in order to minimize the slight risk of cancer from long-term exposure to DBPs. However, diarrheal disease in the developing world is still a leading cause of infant and under-5 mortality and morbidity. In these populations, the risk of death or delayed development in early childhood from diarrheal disease transmitted by contaminated water is far greater than the relatively small risk of cancer in old age.
CDC has tested Safe Water System water to measure the concentration of THMs in the finished water. In that study, household chlorination of turbid and non-turbid waters did not create THM concentrations that exceeded health risk guidelines , . In addition, ceramic filtration, sand filtration, cloth filtration, and settling and decanting were not effective mitigation strategies to reduce THM formation. Since this finding may not hold for all source waters worldwide, reducing organic matter in turbid source water may reduce the potential for DBP formation . To do this:
- Let the water settle for 12-24 hours and then decant water into a second bucket. Chlorinate this decanted water, and/or
- Filter the water through a cloth or filter before chlorination.
The Safe Water System is a proven intervention that consistently reduces diarrheal disease(https://www.cdc.gov/safewater/data/publications-by-topic.html#diarrheal) incidence among users in the developing world. This disease reduction leads to healthier children and adults. There is a slight risk to the ingestion of THMs at the WHO guideline value level. Although the risk from THMs is important to address, until centrally treated, piped water can be delivered to every family, the initial critical need is the provision of microbiologically safe drinking water to reduce the incidence of diarrhea and other waterborne disease.
If you have any questions or comments on this page or the Safe Water System, please email
References
- White, G. The Handbook of Chlorination, 2nd Edition. Van Nostrand Reinhold Company, New York. 1986.
- Gordon G, Cooper WJ, Rice RG, Pacey GE. Disinfectant residual measurement methods. AWWA Research Foundation, American Water Works Association. 1987.
- Calderon RL. The epidemiology of chemical contaminants of drinking water. Food Chemical Toxicology. 2000;38:S13-S20.
- Rook JJ. Formation of haloforms during chlorination of natural waters. Water Treatment Examination. 1974;23:234-243.
- Richardson SD. The role of GC-MS and LC-MS in the discovery of drinking water disinfection by-products. Environmental Monitoring. 2002;4(1):1-9.
- Lin, Tsair-Fuh, Shih-Wen Hoang. Inhalation exposure to THMs from drinking water in south Taiwan. Science Total Environment. 2000;246:41-49.
- Backer, LC, Ashley DL, Bonin MA, Cardinali FL, Kieszak SM, and Wooten JV. Household exposures to drinking water disinfection by-products: whole blood trihalomethanes levels. J Expo Anal Environ Epidemiology. 2000;July-August 10(4); 321-6.
- WHO. Guidelines for drinking-water quality, 2nd edition, Volume 2: Health Criteria and other supporting information[PDF – 94 pages]. World Health Organization, Geneva. 1996.
- WHO. Guidelines for drinking-water quality, 2nd edition, Volume 1: Recommendations. World Health Organization, Geneva. 1993.
- WHO. Guidelines for drinking-water quality, 4th edition. World Health Organization, Geneva. 2011.
- EPA. National primary drinking water standards.
- EPA. Comprehensive disinfectants and disinfection byproducts rules (Stage 1 and Stage 2): Quick reference guide. 2010.
- EPA. Integrated Risk Information System.
- Lantagne DS, Blount BC, Cardinali F, Quick R. Disinfection by-product formation and mitigation strategies in point-of-use chlorination of turbid and non-turbid waters in western Kenya. J Water Health. 2008;6(1):67-82.
- Lantagne DS, Cardinali F, Blount BC. Disinfection by-product formation and mitigation strategies in point-of-use chlorination with sodium dichloroisocyanurate in Tanzania. Am J Trop Med Hyg. 2010;83(1):135-43.

















